44 fda structured product labels
UMLS Metathesaurus - MTHSPL (FDA Structured Product Labels) - Synopsis Authority The U.S. National Library of Medicine (NLM) produces the Metathesaurus FDA Structured Product Labels (MTHSPL), which is based on the Food and Drug Administration (FDA) Structured Product Labeling (SPL). Information for this source is extracted from the NLM DailyMed Web site. Purpose FDA Label Search-Company Name 10903 New Hampshire Avenue. Silver Spring, MD 20993. Ph. 1-888-INFO-FDA (1-888-463-6332) Contact FDA. For Government. For Press.
Licensed | FDA This terminology is related to the Wholesale Drug Distributor and Third-Party Logistics Provider reporting initiative. ... Structured Product Labeling Resources Business Entity Identifiers;

Fda structured product labels
UMLS Metathesaurus - MTHSPL (FDA Structured Product Labels) - Metadata Metathesaurus FDA Structured Product Labels, 2022_02_25: Short Name: FDA Structured Product Labels: Family: MTHSPL: Metathesaurus Insertion Version: 2022AA: Restriction Level: 0: Language: ENG: Context Type: License Contact: RxNorm Customer Service U.S. National Library of Medicine 8600 Rockville Pike Bethesda MD United States › vaccines › programsIIS COVID-19 Vaccine Related Code | CDC The following vaccines and associated tradenames have been approved by the FDA under BLA License. They are listed separately because while they may represent the same formulations as the EUA authorized and labeled products listed above, the NDCs listed with the new BLA licensed tradenames in the FDA BLA approval or the FDA Structured Product Labels (SPL) are not currently being produced by the ... NaturesPlus Supplements May Cause "Severe Reactions" — Best Life May 09, 2022 · On May 6, the FDA announced that Natural Organics had issued a voluntary recall of specific packages of its NaturesPlus Keto Living Sugar Control Capsules 90 count supplements. According to the notice, the affected products come packaged in white jars with black caps and labels with orange, white, and black printing.
Fda structured product labels. Structured Product Labeling Improves Detection of Drug-Intolerance Issues Introduction and Objective. The HL7 Structured Product Labeling (SPL) standard 1 implemented by the FDA uses the HL7 Reference Information Model (RIM) 2 to represent the chemical and physical nature of medical products and their safe and effective use. While not all of this content is available today, we enrich the 3704 available SPLs with knowledge from the SPL terminology sources, including ... IIS COVID-19 Vaccine Related Code | CDC The following vaccines and associated tradenames have been approved by the FDA under BLA License. They are listed separately because while they may represent the same formulations as the EUA authorized and labeled products listed above, the NDCs listed with the new BLA licensed tradenames in the FDA BLA approval or the FDA Structured Product Labels (SPL) are not … Comirnaty Not Available - by Warner Mendenhall NDCs are listed per FDA Structured Product Label (SPL) document for the BLA licensed product. ... Pfizer has provided the following statement regarding the COMINARTY branded NDCs and labels: "Pfizer received FDA BLA license on 8/23/2021 for its COVID-19 vaccine for use in individuals 16 and older (COMIRNATY). At that time, the FDA published a ... Reed Tech | Best-In-Class Information-Based Solutions and Services Jun 06, 2022 · Build and submit UDI records electronically in Structured Product Labeling (SPL) format or seek subject-matter expertise. Manage Medical Device Product Data for UDI & Syndication Serving the medical device manufacturing industry in the areas of compliance, data management and regulatory requirements experience.
2011AA FDA Structured Product Labels Source Information IMPRINT_CODE. FDA Structured Product Label imprint attribute for code. 14314. ANDA. Abbreviated New (Generic) Drug application number for the MTHSPL drug. 11049. BLA. Therapeutic Biologic Applications number for the MTHSPL drug. Pfizer quietly admits it will NEVER manufacture FDA-approved … Jun 10, 2022 · The following vaccines and associated tradenames have been approved by the FDA under BLA License. They are listed separately because while they may represent the same formulations as the EUA authorized and labeled products listed above, the NDCs listed with the new BLA licensed tradenames in the FDA BLA approval or the FDA Structured Product … 2010AA FDA Structured Product Labels Source Information Metathesaurus Scope MTHSPL includes drug product and active substance terminology used in Structured Product Labels. MTHSPL contains approximately 22,597 drug products and 3,576 substances. Metathesaurus Update Frequency MTHSPL is updated in each Metathesaurus release as part of RxNorm . › dailymedDailyMed Sep 15, 2021 · The National Library of Medicine (NLM)’s DailyMed searchable database provides the most recent labeling submitted to the Food and Drug Administration (FDA) by companies and currently in use (i.e., "in use" labeling). DailyMed contains labeling for prescription and nonprescription drugs for human and animal use, and for additional products ...
PPTX Structured Product Labeling Overview Content of Labeling Product Data Elements Product Name Dosage Form Route of Administration Ingredient (active/inactive/adjuvant) DEA Schedule Product characteristics (color, shape, size, etc…) Packaging Marketing Information (category, status, start and end dates) Representative samples of carton/container labels DailyMed - Download All Drug Labels Full Releases. Warning: The full human prescription and OTC archive files, dm_spl_release_human_rx.zip and dm_spl_release_human_otc.zip, are no longer available due to size considerations.Instead, these archives have been split into multiple parts. The remainder archive files consist of bulk ingredient labels, vaccine labels, and some labels for medical … FDALabel: Full-Text Search of Drug Product Labeling | FDA The FDALabel Database is a web-based application used to perform customizable searches of over 140,000 human prescription, biological, over-the-counter (OTC), and animal drug labeling documents.... MTHSPL (FDA Structured Product Labeling) Source Information AuthorityThe U.S. National Library of Medicine (NLM) produces the Metathesaurus FDA Structured Product Labels (MTHSPL), which is based on the Food and Drug Administration (FDA) Structured Product Labeling (SPL). Information for this source is extracted from the NLM DailyMed Web site.
Drug Labeling Overview - Food and Drug Administration Drug manufacturers and distributors submit documentation about their products to FDA in the Structured Product Labeling (SPL) format. The openFDA drug product labeling API returns data from this...
FDA SPL - Structured Product & Drug Labeling Composition Process | Reed ... Structured product labeling for both prescription and over-the-counter (OTC) drugs must incorporate an overview of the scientific information needed for the correct and effective use of the drug. The labeling is broken up into sections including explanations for use (prescription drugs) or purpose (OTC drugs), adverse effects, and more.
Structured Product Labeling - Food and Drug Administration Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. The openFDA...
› industry › fda-data-standards-advisoryStructured Product Labeling Resources | FDA Apr 06, 2022 · The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information.
Document Type including Content of Labeling Type | FDA loinc code. loinc name. 98075-5: animal cells, tissues, and cell and tissue based product label: 77647-6: animal compounded drug label: 86445-4: blanket no changes certification of product listing
Assessing the Impact of HL7/FDA Structured Product Label (SPL) Content ... Methods. All 2217 labels available on DailyMed were downloaded as of February 23, 2007 and fed into an open-source HL7 v3 based data-infrastructure (HL7 JavaSIG). 12 The HL7 Reference Information Model (RIM) 13 is used directly as the object model. The HL7 RIM represents medicines, packages and ingredient-substances as (physical) Entities related to each other through Roles which specify the ...
eCFR :: 21 CFR Part 314 -- Applications for FDA Approval to … 505(b)(2) application is an NDA submitted under section 505(b)(1) of the Federal Food, Drug, and Cosmetic Act for a drug for which at least some of the investigations described in section 505(b)(1)(A) of the Federal Food, Drug, and Cosmetic Act and relied upon by the applicant for approval of the NDA were not conducted by or for the applicant and for which the applicant has …
dailymed.nlm.nih.gov › dailymed › spl-resources-allDailyMed - Download All Drug Labels Full Releases. Warning: The full human prescription and OTC archive files, dm_spl_release_human_rx.zip and dm_spl_release_human_otc.zip, are no longer available due to size considerations.
Indexing Structured Product Labeling | FDA Center for Drug Evaluation and Research Center for Biologics Evaluation and Research This guidance explains that FDA's Center for Drug Evaluation and Research (CDER) and Center for Biologics...
labels.fda.govFDA Label Search The device labeling has been reformatted to make it easier to read but its content has not been altered nor verified by FDA. The device labeling on this website may not be the labeling on currently...
SPL Xforms | FDA To register, please submit the following information via e-mail to spl@fda.hhs.gov: Attendee's first and last name. Name of your organization. E-mail address. Session name and date of training ...
› industry › structured-product-labelingNSDE | FDA Mar 31, 2022 · In this section: Structured Product Labeling Resources ... this file is generated from SPL documents sent to FDA for inclusion in the FDA Online Label Repository at labels.fda.gov. The information ...
Structured Product Labeling Resources | FDA Apr 06, 2022 · The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information.
DailyMed Sep 15, 2021 · The National Library of Medicine (NLM)’s DailyMed searchable database provides the most recent labeling submitted to the Food and Drug Administration (FDA) by companies and currently in use (i.e., "in use" labeling). DailyMed contains labeling for prescription and nonprescription drugs for human and animal use, and for additional products such as medical …
Structured Product Labeling (SPL) | Data Conversion Laboratory Structured Product Labeling (SPL) is a standard used by the FDA community to facilitate the communication of drug labeling data reliability among various groups such as the FDA, hospitals, prescribing organizations, doctors, and the general public. SPL is an HL7 and ANSI approved standard.
MTHSPL (FDA Structured Product Labels) - Statistics FDA Structured Product Label imprint attribute for shape text: 18077: BLA: Therapeutic Biologic Applications number for the MTHSPL drug: 15324: NDA: New Drug Application number for MTHSPL drug: 11751: DCSA: Controlled Substance Act designation code (e.g. 0,2,3n) 7193: MARKETING_EFFECTIVE_TIME_HIGH:

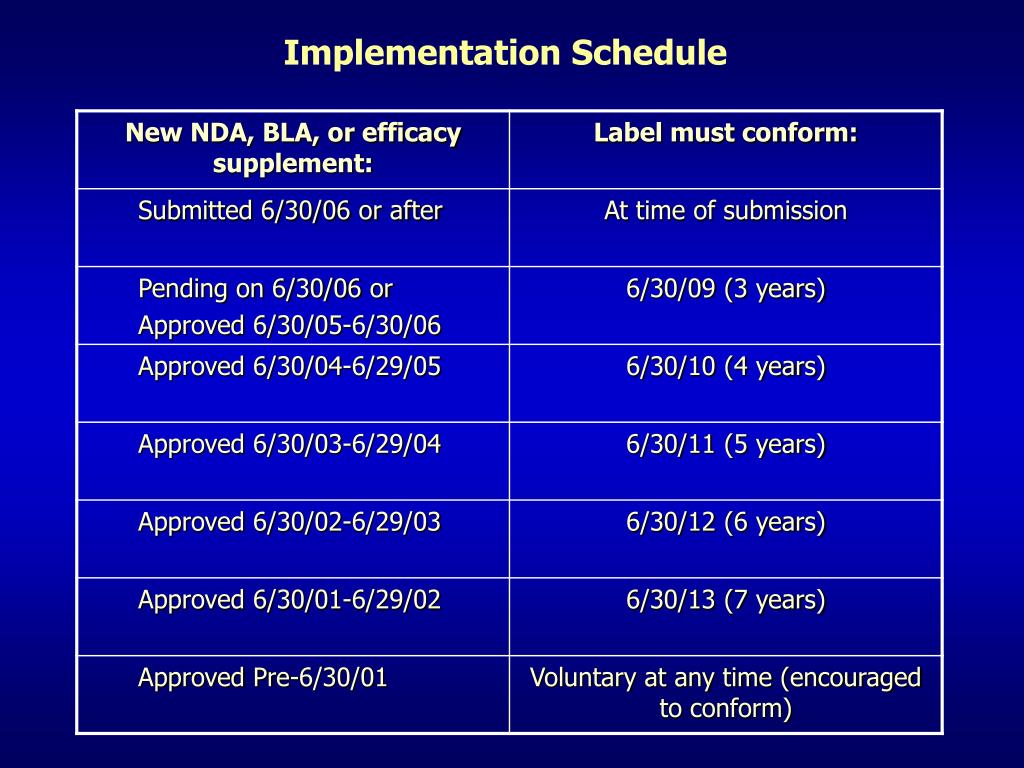







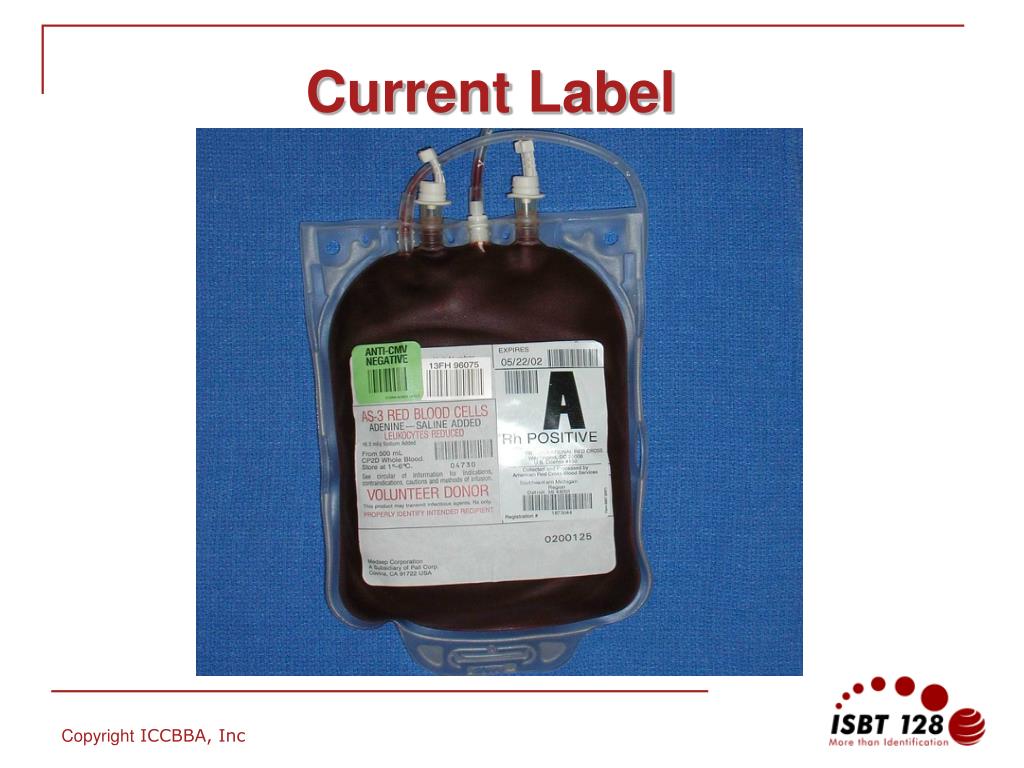
Post a Comment for "44 fda structured product labels"